The SCIENCE CORNER
We are so busy doing research on OMS, we don't take enough time out to explain what we do. Parents frequently ask, "What's the latest on your research?" They have encouraged us to update our web site with highlights from our new data. (By the way, I will often correct them: "It's not my research, but your research.") We are also presenting new results at scientific meetings and publishing them in scientific and medical journals.

[Photo: Dr. Pranzatelli presenting first chemokine data in OMS at the 36th Annual Child Neurology Society Meeting,
Comprehensive biomarkers laboratory
The newest phase of work is creation of a comprehensive biomarkers laboratory. We've kept a bio-repository of blood or spinal fluid specimens for years, so we can go back and test for new biomarkers that weren't known back when the samples were collected. We can now assay hundreds of OMS specimens and have produced new discoveries.
What is a biomarker?
A biomarker is a substance that can be reliably measured and tells us something important about the underlying disease. It is often a body fluid. The better the biomarker, the closer it takes us to the root cause of a disease, thereby providing a target for therapy.
What are the types of biomarkers?
There are three main kinds of biomarkers. The first is a diagnostic biomarker. So far, none has been found for OMS. The second is a biomarker of disease activity. Those we have, and we're looking for more. We discovered B cell expansion in the spinal fluid. Now we have found elevation of certain proteins that recruit B cells into the brain. The third is a treatment biomarker to determine whether a specific medical therapy actually normalizes the biomarker. We now have treatment biomarkers.
Why search for biomarkers in OMS?
OMS comes in different forms and severity levels. Abnormalities can be mild on the one hand, and require little treatment, or devastating on the other, requiring multiple and prolonged immunotherapeutic agents. Some children have mostly motor problems, whereas, others have a preponderance of behavioral problems. In some cases a tumor is found; not so in others. Sometimes infants are affected, other times toddlers or pre-school-age children. Some patients do not have relapses, while a subgroup has multiple relapses. Why the differences? Biomarkers are needed to provide an explanation.
Also, without biomarkers, the treatment of OMS is based on trial and error rather than evidence-based. When a treatment works, no on is the wiser about why it worked or why another drug failed. Innovative therapies can be introduced more rapidly with greater likelihood of success when they are targeted against biomarkers of disease activity.
Why study spinal fluid?
The spinal fluid, also known as cerebrospinal fluid or CSF, is in direct contact with the brain extracellular fluid. That makes it the best information source we have of what is going on in the brain. The active components and by-products of brain inflammation typically find their way into the CSF. In other autoimmune diseases, like multiple sclerosis or rheumatoid arthritis, affected tissue is available for testing. Also, rodent animal models are available to do studies on the mechanism of the disease. In OMS, we have only fluid samples and the cells, proteins, and chemicals they contain, making CSF testing all the more crucial. Keep in mind that most biomarkers in autoimmune diseases of the brain are found in CSF, not blood.
How do biomarkers identify children
at high risk?
By testing for correlations between biomarker candidates and severity level in children with OMS, we have found that children with severe OMS have multiple biomarker abnormalities. Children with mild OMS typically have fewer or less severe biomarker abnormalities. The NPMC has proposed individualized therapy for OMS based on the biomarker profile. Our data indicate that irreversible brain injury can occur very early on, so those high-risk patients need to be promptly identified.
What biomarkers have been discovered
in OMS?
We have found several biomarkers of disease activity so far: 1) immune cells, 2) chemokines and other cytokines, and 3) autoantibodies.
IMMUNE CELLS
The first was the percentage of CSF B cells. Compared to normal children, in whom the frequency of B cells is < 1%, CSF B cells in children with OMS are typically several-fold higher. The highest we have measured is 29%. B cells contribute to autoimmune disease in two major ways. First they produce autoantibodies, which can damage cells and the connections between cells. Secondly, they present antigen to T cells, which then attack the brain in pursuit of the antigen.
CYTOKINES
Cytokines are small proteins secreted by immune cells or body tissues. Another important biomarker is the cytokine B Cell Activating Factor (BAFF). BAFF is essential to the proliferation and maintenance of B cells. Without BAFF, B cells that gain entry into the CSF and brain would probably die. CSF BAFF levels correlate with CSF autoantibody production in pediatric OMS. [Publication: Pranzatelli MR, et al. Cytokine 44:26-32, 2008]
CHEMOKINES
Cytokines that attract immune cells into inflammed tissues or fluid are termed chemokines, short for chemoattractant cytokines. We found that B cell attractant 1 (BCA-1 or CXCL13) is increased in untreated OMS, making it a likely mechanism for B cell entry into the nervous system. We also found that the CXCR5 receptor it binds to on B cells, is increased in children with OMS. ACTH and, to a lesser extent, steroids are successful in lowering CSF CXCL13 while they are being administered, perhaps accounting for their clinical effect. The illustration shows the structure of a CXC chemokine.
We also discovered that the inflammatory chemokine Interferon-inducible Protein-10 (IP-10 or CXCL10) is increased in the CSF in OMS, and that most treatments for OMS do not normalize it. Better ways to reduce or block CXCL10 may be important for OMS. [Publication: Pranzatelli et al. Annals of Neurology 62(supplement 11): S112, 2007.]
AUTOANTIBODIES
Recently, we found that 35% of children with OMS are positive for CSF oligoclonal bands or OCB. For comparison, CSF OCB are found in 95% of patients with multiple sclerosis. OCB measured commercially are IgG antibodies. They indicate abnormal antibody synthesis within the CSF by B cells.
How have biomarkers improved treatment
and understanding of OMS?
The foundation was laid for use of the anti-B-cell agent rituximab by finding increased CSF B cell frequency (called 'expansion') in OMS. Now, chemokine studies have provided an explanation for what attracts B cells into the CSF and brain. Treatments based on the chemokine/cytokine results are planned. The discovery of increased CSF BAFF and CXCL10 in pediatric OMS has raised attention to possible involvement of the brain astrocyte in OMS. In multiple sclerosis, both cytokines are secreted by astrocytes, and indicate astrocytic gliosis, or ‘scar' formation. Previously in paraneoplastic research, the focus has been entirely on neurons. Astrocytes participate and interact with brain neurons. We are now studying them.
How do we use biomarkers at the NPMC?
Biomarker testing is done on all patients at our center. Lymphocyte subsets and oligoclonal bands are measured by hospital laboratories, either locally or as send-out tests, and billed to insurance. In addition, chemokine and other cytokine biomarkers are measured in Dr. Pranzatelli's laboratory. Those tests are funded by grants and donations and not charged to the patient. We are doing analysis for biomarker 'clusters' that would have the most predictive value in OMS.
How do you test for a biomarker?

[Photo: Nathan R. McGee,
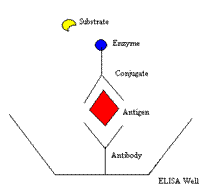
ELISA ASSAY
ELISA is an acronym for Enzyme-Linked Immunosorbent Assay. The assay, which takes all day to perform, begins with a 96-well plate that is purchased as a kit. Each well comes pre-coated with an antibody to the cytokine or chemokine to be tested. If the cytokine (antigen) is present in the serum, plasma, or CSF sample, it binds to that antibody. To detect the cytokine, it is then labeled with the anti-cytokine antibody to which an enzyme is attached. That enzyme is reacted with a substrate, producing a color change reaction that can be quantitated. The principal of the multi-step assay is shown here.
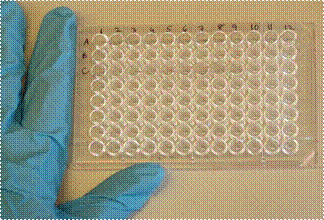
The first 16 plate wells (arranged as two columns on the left) consist of 1 blank and 7 standards arranged from low to high concentration and run in duplicate. The standards (cytokine supplied by the manufacturer supplies) are crucial, because all the samples from children with OMS are compared to them.
Patient samples must be kept frozen before use and thawed slowly in cold water. They are pipetted in duplicate wells for accuracy. The plate is incubation (2 hours) on a slow shaker speed to facilitate maximum binding of cytokine. The sample is washed out of the wells, so only bound cytokine remains. Conjugate, which is the same antibody coated to the wells except with another molecule (enzyme) attached, is then added, and another incubation (1-2 hours) begins.

After another wash cycle, substrate is added and binds to enzyme on the conjugate. The liquid in the well turns blue. There is no color change if cytokine is not present in the patient sample. [The first two columns are the blanks and the standards, which are arranged from lowest to highest concentration. Patients in columns 4 and 9 have the highest cytokine concentrations, and those with intermediate concentrations are found in several other rows.]

Following a third incubation (30 minutes), Stop Solution is added. Stop solution halts the color change of the substrate. The color of liquid in the wells changes from blue to yellow in patient samples with detectable cytokine levels.

To quantify the cytokine, a Plate Reader (spectrophotometer) uses light absorption to measure the color of the liquid in the wells. The darker the color, the more cytokine (protein) is present in the sample. The photograph shows the plate situated in the reader.

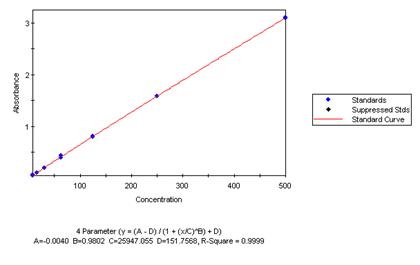
Here is an example of a Standard Curve, showing the low to high concentration standards from left to right. The linearity of the graph and closeness of the duplicates illustrate Nathan’s excellent laboratory skills.
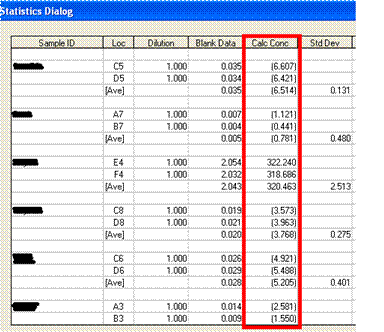
The computer output illustrates that patient samples E4 and F4 have a very high cytokine level compared to the other patient shown on that page of the report.
What is the future of biomarker
research in OMS?
We are very proud to introduce a new approach to OMS, and look forward to sharing more research results with you in the near future.